Thermoreversible Gelation of Poly(ether ether ketone)
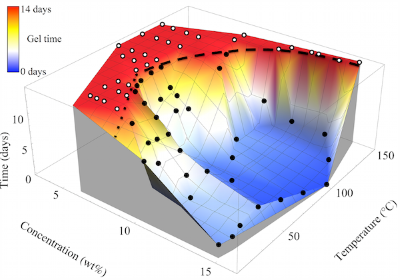
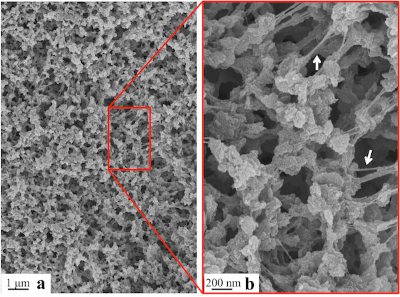
Although gels containing a liquid solvent are common soft materials for a variety of applications, a growing field is concerned with low-density gels containing air instead of liquid, known as aerogels. The lightweight, high surface area/porosity, and generally low thermal conductivity inherent to aerogels makes them desirable material candidates for thermal insulation, filtration processes, and even low dielectric constant materials. Aerogels are typically prepared from solvent containing gels via freeze drying or supercritical drying. The most widely studied aerogel materials are chemically cross-linked inorganic silicates; although recently, a few purely organic polymer aerogels formed from super-critical CO2 extracted semicrystalline thermoreversible gels have been reported. Here, we demonstrate the first formation of a monolithic, thermoreversible gel of PEEK (without a dense surface layer) in dichloroacetic acid (DCA) over a wide range of temperatures and concentrations. Moreover, we demonstrate a facile solvent exchange process for yielding the first reported hydrogels and aerogels of PEEK composed of submicron morphological features and an average pore size on the order of 10 nm.
1. Talley, S. J.; Yuan, X.; Moore, R. B., “Thermoreversible Gelation of Poly(ether ether ketone),” ACS Macro Lett., 2017, 262-266.
2. Talley, S. J.; Vivod, S. L.; Nguyen, B. A.; Meador, M. B.; Radulescu, A.; Moore, R. B.; “Hierarchical Morphology of Poly (ether ether ketone) Aerogels”, ACS Appl. Mater. Interfaces 2019
3. Talley, S. J.; AndersonSchoepe, C. L.; Berger, C. J.; Leary, K. A.; Snyder, S. A.; Moore, R. B.; “Mechanically robust and superhydrophobic aerogels of poly(ether ether ketone)”, Polymer, 2017
Gel-State Polymer Chemistry
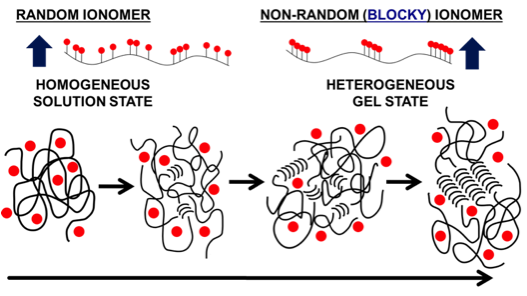
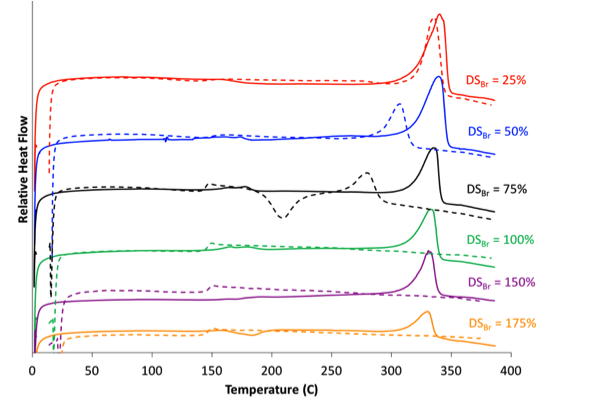
Functionalized poly(ether ether ketone) (PEEK), primarily sulfonated PEEK (SPEEK), has long been regarded as a promising candidate for ion exchange membranes due to its low cost, excellent mechanical properties, and ease of production. SPEEK is conventionally prepared by direct sulfonation of PEEK pellets in sulfuric acid, which affords little control over functional group sequencing and results in membranes with large distributions in both the number and location of ionic groups. Our group focuses on obtaining better control of functional group placement in PEEK to determine the influence of ionic architecture on membrane properties. By utilizing a novel solvent for PEEK that also enables gelation (see above), we are now able to produce SPEEK with a random architecture (functionalized in the solution state) or with a blocky architecture (functionalized in the gel state). By blocking up the ions, we are able to maintain the crystallinity of PEEK, which not only improves the mechanical properties of the resultant membranes but also drives phase separation into well-ordered domains, as compared to random analogues. Furthermore, we have successfully extended this work to the halogenation of PEEK, which may be used as a handle for subsequent functionalization of the PEEK.
1. Anderson, L. J.; Yuan, X.; Fahs, G. B.; Moore, R. B., “Blocky ionomers via sulfonation of poly(ether ether ketone) in the semicrystalline gel state”, Macromolecules, 2018
2. Noble, K. F.; Noble, A. M.; Talley, S. J.; Moore, R. B., “Blocky bromination of syndiotactic polystyrene via post-polymerization functionalization in the heterogeneous gel state”, Polymer Chemistry, 2018
3. Anderson, L. J.; Moore, R. B.; “Sulfonation of blocky brominated PEEK to prepare hydrophilic-hydrophobic blocky copolymers for efficient proton conduction”, Solid State Ionics 2019
Morphology-Processing Relationships of Perfluorinated Ionomer Membranes for Fuel Cells

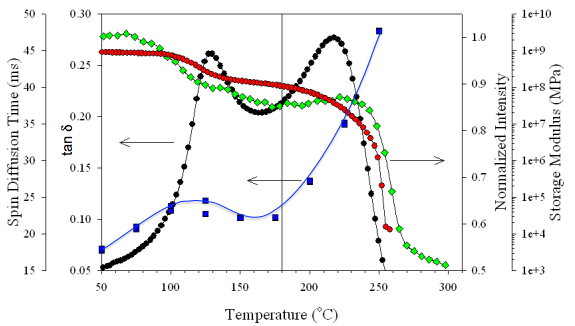
Perfluorosulfonated ionomers (PFSIs) are well-known for their chemical stability and physical properties due to the resulting morphology of interactions between their PTFE backbone and ionic side chains. This morphology leads to remarkable proton conductivity and chemical properties that have garnered widespread use of PFSIs as membranes in fuel cell membrane electrode assemblies (MEAs).2 While Nafion has been the benchmark PFSI since its creation in the late 1960s, new perfluorinated ionomers with different sid chain structures have been created in an effort to optimize functionality while maintaining mechanical stability. Finding a balance between functionality and mechanical stability is critical for developing the best ion exchange membranes, and the best place to start is by probing the morphology of these ionomers controlled by differing side chain structure. Our group focuses on analyzing new perfluorinated ionomers to gain an understanding of their morphology and the best processing conditions for application in next generation fuel cells.
1. Page, K. A.; Cable, K. M.; Moore, R. B., Molecular Origins of the Thermal Transitions and Dynamic Mechanical Relaxations in Perfluorosulfonate Ionomers. Macromolecules 2005, 38, 6472.
2. Mauritz, K. A.; Moore, R. B., State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535.
Blends of Poly(ethylene terephthalate) (PET) and MXD6 for Packaging Applications
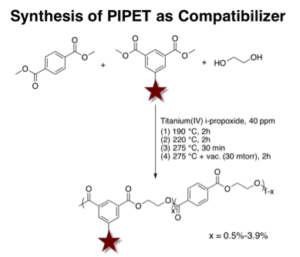
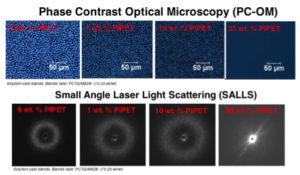
Poly(ethylene terephthalate) (PET) is widely used in the packaging industry. The oxygen barrier properties of PET are acceptable for many food and beverage products but do not meet the stringent requirements for packaging highly oxygen-sensitive food and beverage products. Blending PET with aromatic polyamides, such as MXD6, reduces the inherent oxygen permeability of the polyester matrix. However, due to the immiscibility of these two parent polymers, a compatibilizer is necessary to achieve efficient and stable mixing. Herein, we present the influence of polyester ionomers, PIPET, as a minor-component compatibilizer on the morphology and properties of PET/MXD6 blends. Using phase-contrast optical microscopy, we demonstrate decreased phase size of the dispersed MXD6 component as a result of added polyester ionomers. Consistent with previous literature results, this compatibilization behavior is attributed to specific interactions between the ionic functionality and the amide units of MXD6, which lowers the interfacial tension and leads to a reduction in phase dimensions.
1. Ju, L.; Dennis, J. M.; Heifferon, K. V.; Long, T. E.; Moore, R. B.; “Compatibilization of Polyester/Polyamide Blends with a Phosphonated Poly (ethylene terephthalate) Ionomer: Comparison of Monovalent and Divalent Pendant Ions”, ACS Appl. Polym. Mater. 2019
2. Ju, L.; Pretelt, J.; Chen, T.; Dennis, J. M.; Heifferon, K. V.; Baird, D. G.; Long, T. E.; Moore, R. B., “Synthesis and characterization of phosphonated Poly (ethylene terephthalate) ionomers”, Polymer, 2018